Service advantages
Professional Guidance
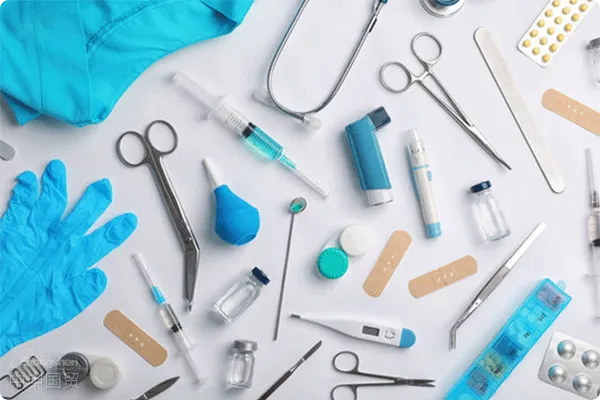
Common Issues in Importing and Exporting Medical Devices
Imported medical devices must be equipped with Chinese instructions and Chinese labels. The content needs to meet the requirements of relevant mandatory standards, and clearly indicate the place of origin of the medical device and the legal person information in China.
Agency Services for Class I and Class II Medical Device Products
Contact Us
If you want to know more information about foreign trade import and export agency, you can leave a message in the form, and we will contact you in a timely manner.

service@sh-zhongshen.com

139-1787-2118

13917872118












